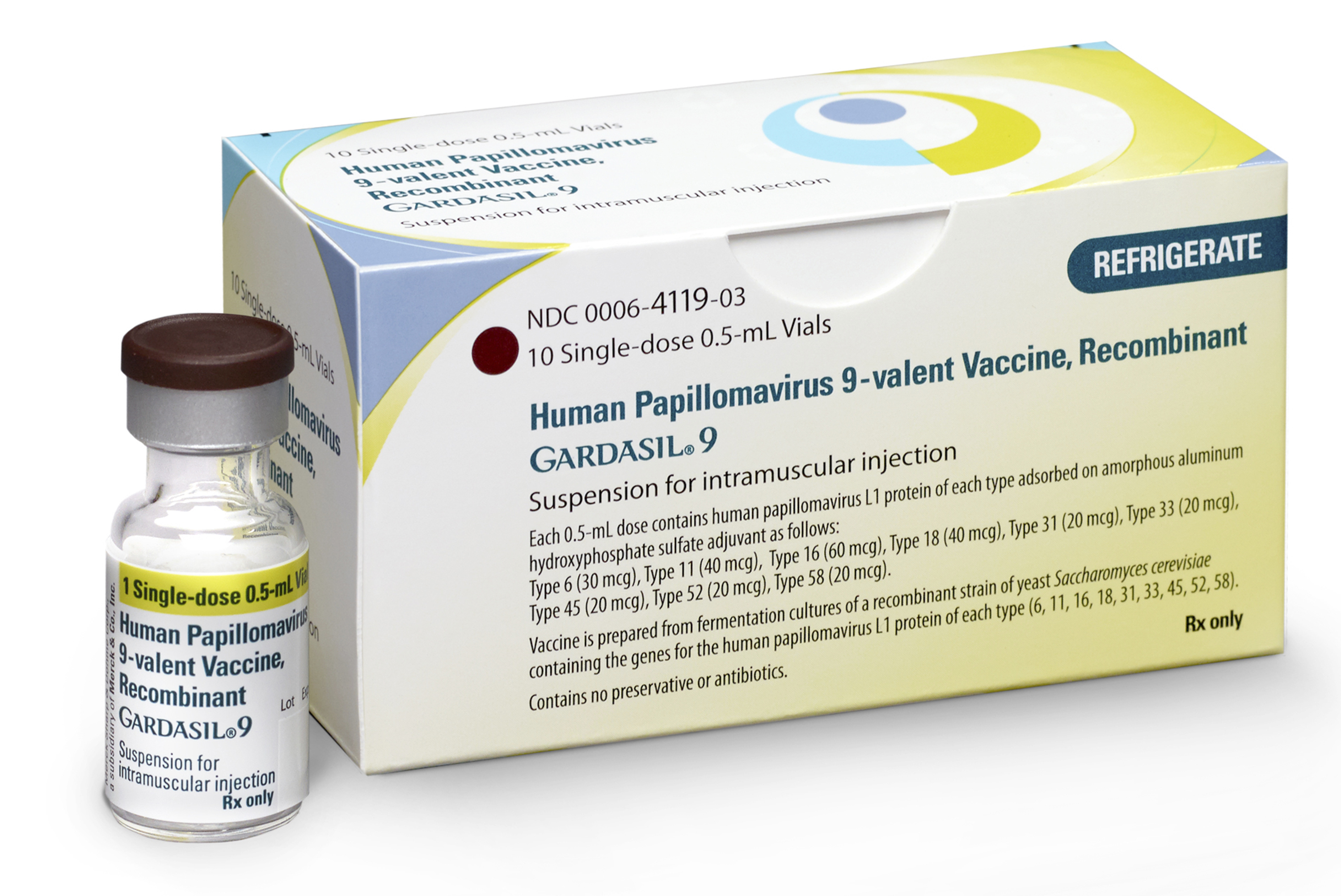
U.S. regulators have expanded the use of Merck’s cervical cancer vaccine to adults up to age 45. The vaccine was previously only for preteens and young adults through 26. The Food and Drug Administration on Friday approved use of Gardasil 9 (GARR’-duh-sill) for women and men through 45.
Videos by Rare
The vaccine protects against the human papilloma virus — or HPV — which can cause cervical cancer, certain other cancers and genital warts. The virus is very common and is spread through sex. The shots are particularly recommended for boys and girls before they first have sex and could get infected. Research shows that the vaccine also protects older adults, too. Merck said the list price for Gardasil 9 is $205 per dose. Two or three doses are needed.
By LINDA A. JOHNSON, AP Medical Writer